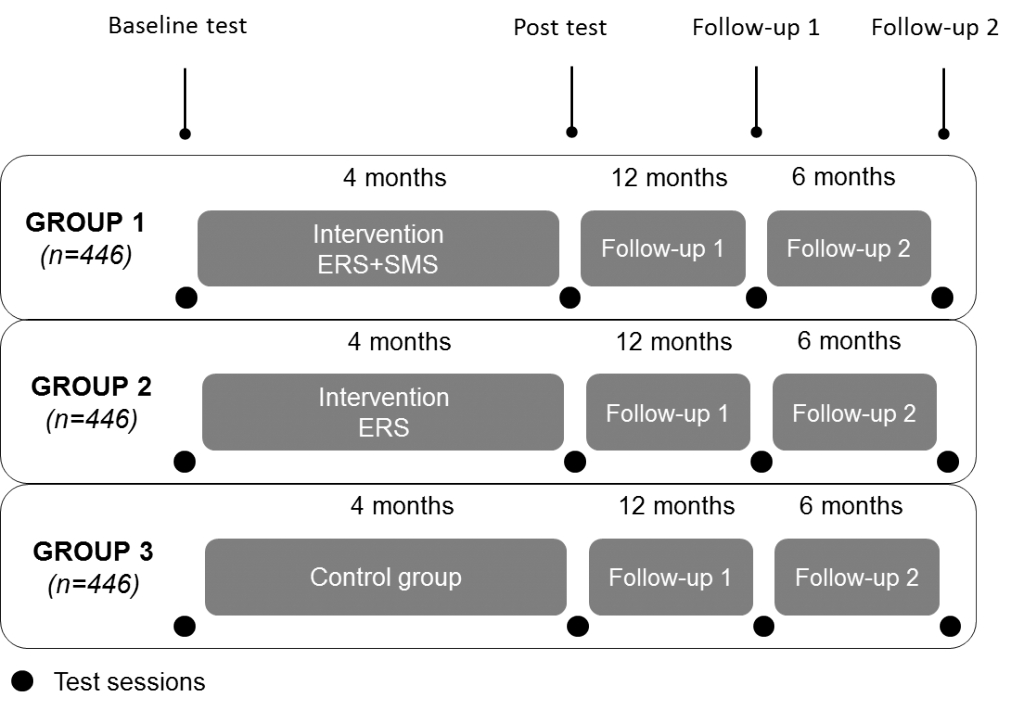
A complex intervention consisting on an Exercise Referral Schemes intervention with Self-Management Strategies will be delivered in primary care or community settings in urban areas of Catalonia – Spain (FB-FSIE), Syddanmark – Denmark (SDU), Baden-Württemberg – Germany (UULM) and Northern Ireland – UK (QUB). Each site will recruit around 335 participants randomized into three groups:
- 1.ERS+SMS
- 2.ERS
- 3.healthy lifestyle advice in usual care (control)
1. Primary outcomes
- Physical activivty & sedentary behaviour (Actigraph, ActiPal, Axivity, SBQ)
2. Secondary outcomes}
- Physical function (SPPB & 2-MWT)
- Muscle function (Linear encoder & grip strength)
- Cost-effectiveness analysis (Interview)
- Anthropometry (Bio-impedance, waist and hip circumference)
- Self-rated health and health-related quality of life (SF-12, EUROQOL-5D, ICECAP-O)
- Activities of Daily Living (6-item questionnaire)
- Anxiety and Depressive symptoms (Hospital Anxiety and Depression Scale)
- Social network and loneliness (Lubben Social Network Scale-6)
- Physical activity self-regulation (12-item Physical Activity Self-Regulation Scale)
- Physical fatigue (Pittsburg Fatigability Scale)
- Self-Efficacy for exercise (Marcus’s Self-Efficacy Questionnaire)
- Falls and Fear of falling (Short Falls Efficacy Scale – International)
- Disability (Short form Late Life Function and Disability Index)
- Cognitive function (TMT, SF-MMSE)
- Blood samples (IL-6, hsCRP, TNF-alpha, IGF-1.)
- Biopsies (Myostatin, IL-6, IL-8, IL-15, VEGF, BDNF, FGF21, irisin, myostatin, Type 2/Type 1 fibre ratio, Wnt and Notch signaling, CDC42)
You can download the study protocol (Giné-Garriga et al 2017 – The SITLESS project) from the sitless webpage – (Link)
